Training session on Adverse Outcome Pathways with OECD experts
Kicking off the training – Mirjam Luijten from RIVM
Adverse Outcome Pathways (AOPs) are a key deliverable for the regulatory uptake of the ALTERNATIVE project. But what do AOPs exactly represent? Which purpose do they serve? And how are they developed? In order to develop a common understanding and answer these questions, the ALTERNATIVE consortium invited members of the OECD Education, Training and Outreach subgroup of the AOP Framework for a training session on the basic principles of AOPs, which took place online on 17 December 2021.
Mirjam Luijten (RIVM, Netherlands) kicked off the training by explaining the need to structure the large amount of available data into AOPs to make them usable for regulatory, predictive toxicology.
Basic AOP concepts presented by Brigitte Landesmann (JRC/EURL-ECVAM, European Commission, Italy)
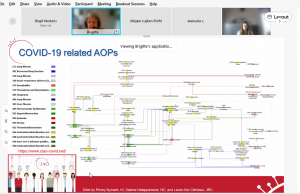
Brigitte Landesmann (JRC/EURL-ECVAM, European Commission, Italy) presented the basic concept of AOPs as a living knowledge base for stressor-independent pathways. AOPs have a modular structure in which molecular initiating events (MIEs), cellular and tissue level key events (KEs) and regulatory relevant adverse outcomes (AOs) are linked by specific key event relationships (KERs). She also discussed some examples of AOPs and how they can be joined into AOP networks, which are suitable for mixture assessment, and more.
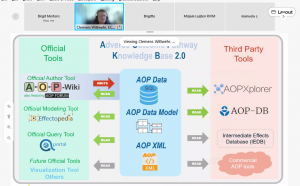
AOP databases presented by Clemens Wittwehr (JRC/EURL-ECVAM, European Commission, Italy)
Clemens Wittwehr (JRC/EURL-ECVAM, European Commission, Italy) presented the AOP databases, their structure, their use and the related tools made available by the OECD. Notably also the AOP forum shall serve for a user-driven information exchange.
Bette Meek (University of Ottawa, Canada) zoomed in on the Weight of Evidence Characterization for AOPs. Conclusions are required in terms of low, moderate or high evidence for biological plausibility and empirical support for KERs and for the essentiality of KEs for the activation of following KEs.
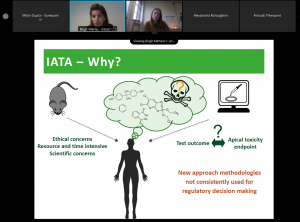
AOPs as a basis for IATAs – Birgit Mertens from Sciensano
Birgit Mertens (Sciensano, Belgium), who is leading the work package for the regulatory uptake within the ALTERNATIVE consortium, described how AOPs can be used as basis for the development of Integrated Approaches to Testing and Assessment (IATAs) and their mechanistic characterization.
Finally, Martin Paparella (Medical University Innsbruck, Austria), also a partner of the ALTERNATIVE consortium contributing to the regulatory work package and member of an expert group of the European Food Safety Agency (EFSA), showed the practical experience of that expert group with the development of an AOP for developmental neurotoxicity. More specifically, they explored the utility of a Bayesian Network-based approach for a probabilistic Weight of Evidence characterization.
In the discussion, a basic common understanding of the AOP concept was gained. Once the AOPs will be progressed within the ALTERNATIVE consortium, specific follow-up discussions will be organized.