ALTERNATIVE presentation at PARERE workshop on regulatory requirements for cardiotoxicity data
On 21st December 2021, a joint workshop of the Austrian and Belgian networks for the ‘Preliminary Assessment of Regulatory Relevance of Alternative methods and approaches (PARERE)’ was organized online. The aim of the workshop was to discuss the data requirements for cardiotoxicity across different regulatory fields.
Participants of the PARERE workshop
The workshop audience included 25 participants representing Austrian and Belgian regulators from different fields as well as partners from the ALTERNATIVE consortium. In order to set the scene, Dr. Nunzia Linzalone (CNR, Italy) started the workshop with a presentation explaining why we need to worry about cardiotoxicity of chemicals. Next, Dr. Sonja Beken (FAGG, Belgium) and Dr. Philippe Castelain (Sciensano, Belgium) provided an overview on the regulatory requirements for cardiotoxicity for pharmaceuticals and pesticides.
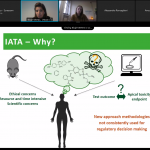
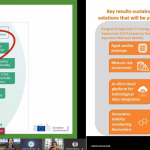
In the second part of the meeting, Dr. Ard Teisman (J&J, Belgium) presented a 2D in vitro model for cardiotoxicity. In his presentation, he highlighted the strengths as well as the limitations of the in vitro model. Moreover, he stressed the importance of considering the development and validation of stem cell based in vitro models for cardiotoxicity in general. Finally, Dr. Birgit Mertens (Sciensano, Belgium) introduced the ALTERNATIVE project, and more specifically its work on regulatory needs.
The presentations led to insightful discussions between the participants. Overall, the workshop provided a good basis for mapping existing regulatory requirements and needs for cardiotoxicity that will be conducted within the ALTERNATIVE project.